Image Details

Caption: Figure 1.
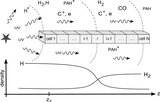
Chemical mechanism used in our computation of H 2 formation. PAH represents any normally hydrogenated PAH (e.g., coronene C 24H 12, ovalene C 32H 14); PAH −H represents the dehydrogenated species where one peripheral hydrogen is missing, while PAH +H represents a PAH with an additional hydrogen atom (e.g., C 32H 15 for ovalene). The + superscript denotes the corresponding cation. Anion chemistry is not included for simplicity. The arrows represent the various processes that transform one PAH into another. Four main processes are considered: (1) ionization with rate constant k ion; (2) recombination between ions and electrons with rate constant k rec and branching ratio a and (1− a) toward H 2 and H formation, respectively; (3) photodissociation with H atom ejection ( k diss) or H 2 ejection ( k' diss); (4) chemistry with H and H 2 addition through radiative association ( k H and k H2).
Copyright and Terms & Conditions
© 2009. The American Astronomical Society. All rights reserved.