Image Details
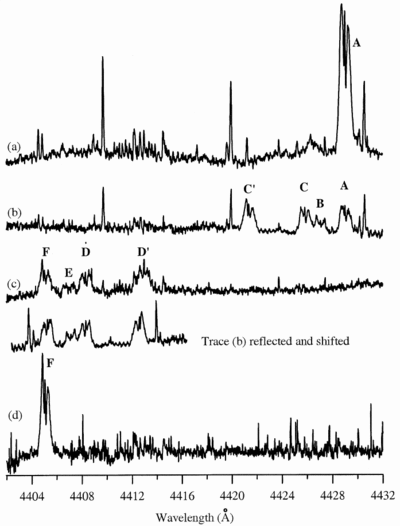
Caption: Fig. 2.
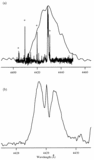
Deuterium isotope shifts of the laboratory band at λ4429. Trace (a) shows the band A produced with normal benzene, the same as that shown in Fig. 1. Trace (b) shows the three singly deuterated isotopic bands B, C, and C﹩\arcmin﹩ observed with C6H5D, and (c) shows the singly hydrogenated bands D, D﹩\arcmin﹩, and E observed with C6HD5. Finally, (d) shows the fully deuterated isotopic band F observed with pure C6D6. To a small fraction of the width of each band, the shifts in (c) are the mirror image of those in (b). The intensity ratio of the residual normal band A relative to band B in trace (b) is an interesting question. It would be expected to be five if the hydrogen atoms were randomly shuffled in the discharge prior to formation of the carrier of our band, but would be only one if the carrier were generated by randomly removing one hydrogen (or deuterium) from C6H5D. The observed ratio of about two is presumably the result of incomplete shuffling. Similarly for the residual fully deuterated band F in (c).
Copyright and Terms & Conditions
© 2000. The American Astronomical Society. All rights reserved. Printed in U.S.A.